Smart Nuclide Announces the License Agreement with Hober Biotech AB For an Innovative HER2 Radioactive Imaging Agent
Suzhou, China and Stockholm, Sweden, January 12, 2022 – Suzhou Smart Nuclide and Hober Biotech AB officially announce today the execution of an exclusive license agreement in Greater China for 99mTc labeled HER-2 target radioactive imaging agent ADAPT6.
ADAPT6 is a unique radioactive imaging agent which can rapidly distinguishes HER2-positive and HER2-negative tumors, HER2 plays an important role in the treatment of patients with breast cancer. Besides, in other solid tumors such as gastric cancer and lung cancer, the expression of HER2 is also very important for both diagnosis and treatment.
“ADAPT6 is well matched with the nuclear medicine pipelines of Smart Nuclide. We always adhere to the principle of “independent research & development as priority, global cooperation as supplement”, and work together to achieve the sustainable development of innovative nuclear medicine.” Said Smart Nuclide’s Chief Executive Officer, and Founder, Xu Tao. “In the future, based on our continuously optimized nanobody technology platforms, we will not only continuously develop radiotherapeutic drugs for mature tumor targets, but we will also build up cGMP workshop and sales teams, to transform from a biotechnology company to a biopharmaceutical company. ”
ADAPT6 was jointly developed by Dr. Vladimir Tolmachev and Dr. Sophia Hober from Uppsala University and KTH Royal Institute of Technology, respectively. ADAPT6 was developed and optimized for decades, and more than 30 high-level SCI papers have been published in related research. This drug has now completed phase I clinical trials. For those untreated patients with primary breast cancer, ADAPT6 is safe with no related adverse events reported according to the research. A PET/CT or SPECT can visualize the expression levels of HER2 in all tumors in vivo in a real-time and dynamical basis, 1-2 hours after the injection of ADAPT6.
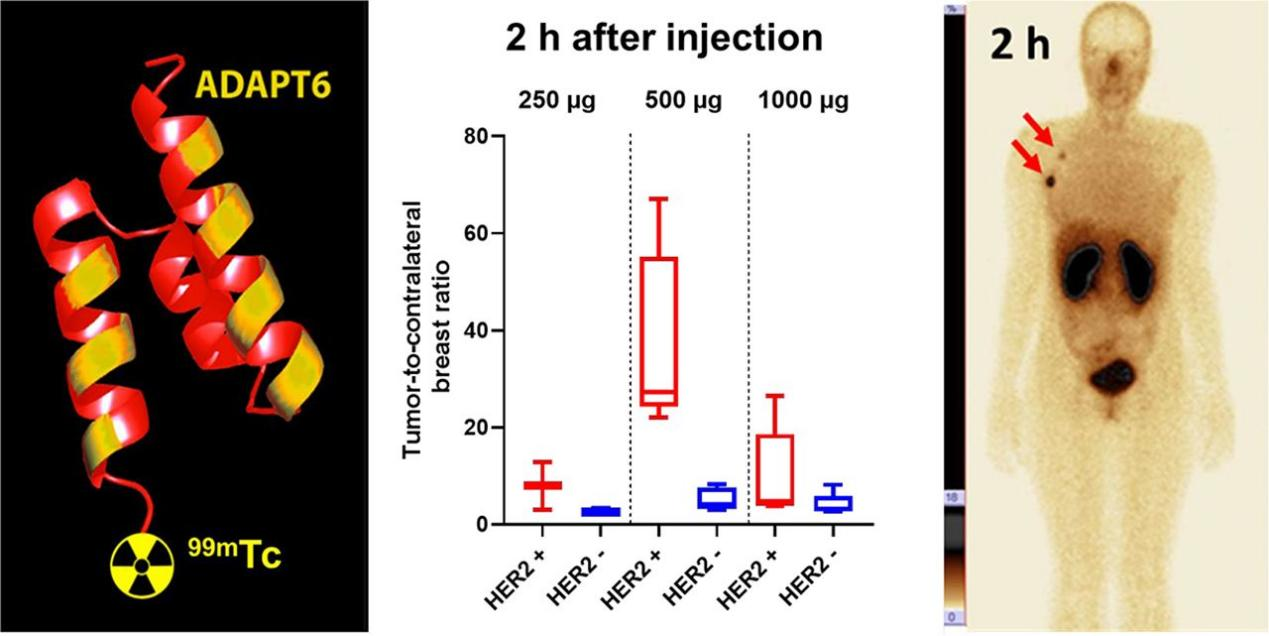
In addition to being applied to the diagnosis and treatment of breast cancer and gastric cancer, the value of HER2 in the diagnosis and treatment of non-small cell lung cancer and bladder cancer is also being recognized. In the future, ADAPT6 will undoubtedly realize early screening and treatment of related cancer through radioactive imaging.
In fact, with the license agreement of ADAPT6, Smart Nuclide’s self-developed radioactive imaging drugs are also advancing rapidly. The company has independently developed the 68Ga labeled imaging agent targeting PD-L1 (SNA002) and the whole-body tumor-infiltrating lymphocyte (TIL) imaging agent (SNA006). Both imaging agents have similar advantages to adapt6 and have great guiding role and development value for immunotherapy.
According to Dr. Xu Tao, SNA002 will soon start the IND application in China and United States, which is expected to be the first radioactive diagnostic drug receives the FDA Clearance of IND application from a Chinese pharmaceutical company. Additionally, Smart Nuclide recently announce the cooperation with The First Affiliated Hospital of Soochow University, conducting a IIT (Investigator Initiated Trial) of SNA006.
